Thyroid Nodule Investigations
The main goal in evaluating a patient with a thyroid nodule is to determine whether their nodule is cancerous. To do so, ultrasound and fine-needle aspiration (FNA) cytology of suspicious thyroid nodules are the mainstays of investigation.
Thyroid Function Tests
Occasionally, thyroid nodules become autonomous (also referred to as “toxic” or “hot”) and produce too much thyroid hormone, resulting in hyperthyroidism (anxiety, difficulty concentrating, fatigue, tremor, heat intolerance, etc). A toxic multinodular goitre contains one or more nodules that are no longer under control of thyroid-stimulating hormone (TSH). A suppressed TSH and normal free thyroxine (fT4) level (compensated hyperthyroidism) is often the first indication that a multinodular goitre or thyroid nodule is becoming autonomous.
Thyroid function tests should be requested for all patients with a suspected nodule in the thyroid gland. Most patients will be euthyroid. If a patient is hyperthyroid, referral to an endocrinologist and/or starting them on carbimazole is usually appropriate. A radionuclide thyroid scan can be helpful in elucidating the function of the nodule(s).
Patients with normal thyroid function tests are sometimes surprised when the diagnosis of papillary thyroid carcinoma is made as they may believe a normal thyroid function test means they will not have thyroid cancer. This is not true. Neither is it true that patients with hyperthyroidism do not have thyroid cancer, although thyroid cancer is very uncommon in patients with thyroid nodules and hyperthyroidism.
Ultrasound Scans
An ultrasound scan is required for all suspected thyroid nodules. It is used to categorise thyroid nodules according to the Thyroid Imaging, Reporting and Data System (TI-RADS) developed by the American College of Radiology.¹² The risk of malignancy increases with a higher TI-RADS classification, as follows:
- TR1 – 0.3 per cent
- TR2 – 1.5 per cent
- TR3 – 4.8 per cent
- TR4 – 9.1 per cent
- TR5 – 35 per cent.
TI-RADS categorises nodules according to their composition (eg, solid, cystic), echogenicity (eg, hypoechoic, isoechoic), shape, margin and echogenic foci (calcification). Points are given for each of these characteristics to determine the TR level. For example, calcification is divided into three categories: coarse calcification (1 point), rim calcification (2 points) and microcalcification (punctate echogenic foci; 3 points). Punctate echogenic foci are commonly seen in thyroid malignancy and need to be distinguished from comet-tail artefacts that are associated with benign colloid nodules.
Recommendations for FNA and ultrasound surveillance are made depending on the size of the thyroid nodule and its TI-RADS classification. For example, FNA is recommended for a 1.5cm TR4 nodule, while ultrasound surveillance is recommended for a 2cm TR3 nodule. A 4cm TR2 nodule requires no follow-up. It is important to remember these recommendations are for asymptomatic euthyroid patients. The figure above gives a good overview of TIRADS and recommendations for subsequent management.
Ultrasound-guided FNA
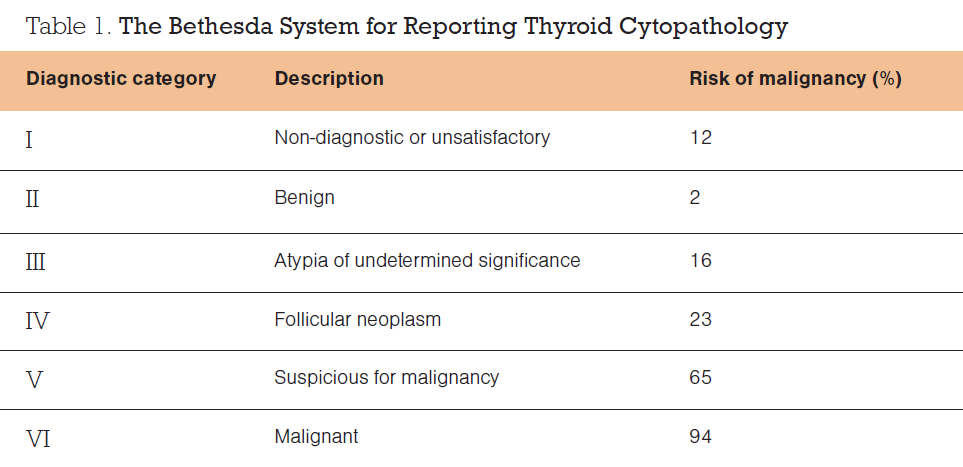
It is recommended that FNA of thyroid nodules is performed under ultrasound guidance. Multiple studies have shown a lower non-diagnostic rate with ultrasound-guided FNA compared with freehand FNA, even for thyroid nodules that are easily palpable. FNA cytology results are classified according to the Bethesda System for Reporting Thyroid Cytopathology,³ as shown in Table 1.
Molecular Analysis
The American Thyroid Association (ATA) guidelines recommend three options for the management of patients with Bethesda III thyroid nodules:⁴
- diagnostic hemithyroidectomy (also called diagnostic thyroid lobectomy)
- close observation (with ultrasound)
- molecular analysis.
Approximately 10 per cent of thyroid FNAs are reported as Bethesda III (atypia of undetermined significance) and a further 10 per cent are reported as Bethesda IV (follicular neoplasm), but only about 16–23 per cent of these patients actually have thyroid cancer.² However, it is common practice to offer surgery to patients with Bethesda III and IV cytology, in the form of a diagnostic hemithyroidectomy. Although this approach detects and treats thyroid cancer in 16–23 per cent of people in this category, 77–84 per cent of people have an operation with no or little therapeutic benefit.
To help further determine which patients are more likely to have cancer, molecular analysis of FNA specimens can be performed by testing the DNA and RNA of the cells obtained. These tests look specifically for mutations in genes associated with thyroid cancer.
The prevalence of some of the common genetic mutations are listed below. Prevalence of genetic mutations in papillary thyroid carcinoma:
- BRAF – 45 per cent
- RAS – 15 per cent
- RET/PTC – 15 per cent.
Prevalence of genetic mutations in follicular carcinoma:
- RAS – 40 per cent
- PAX8/PPARg – 40 per cent.
In the US, Afirma and ThyroSeq are two of the common molecular tests that look at genetic mutations in thyroid cancers. Afirma looks at 593 genes associated with thyroid cancer. Using Afirma, two-thirds of patients with Bethesda III and IV nodules are reclassified as benign.
ThyroSeq looks at 112 genes associated with thyroid cancer. Similarly, ThyroSeq is able to stratify patients with Bethesda III and IV nodules into low, intermediate and high risk of having thyroid cancer. Low-risk patients can be observed. Hemithyroidectomy is recommended for intermediate-risk patients, and total thyroidectomy is recommended for high-risk patients with aggressive types of thyroid cancer.
In New Zealand, ThyroSeq molecular analysis is available, but only if the FNA specimens are sent to Sullivan Nicolaides Pathology in Brisbane, which in turn sends them to Sonic
Healthcare in New York, US. The turnaround time is about four to seven weeks, and the cost to the patient is approximately $2300, which is not covered by health insurance.
In the near future, Idylla ThyroidPrint molecular analysis of FNA specimens of thyroid nodules will be available in New Zealand, with results available in 24 hours. Idylla ThyroidPrint looks at 10 epithelial and stromal cell target genes associated with thyroid cancer. It stratifies risk into low (observe) and high (operate).
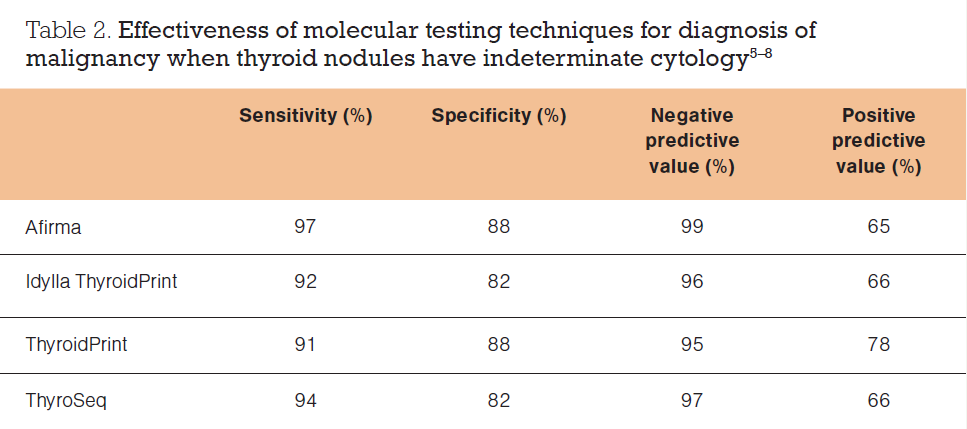
Table 2 shows the diagnostic performance of different molecular tests.⁵⁸ It is important to realise that as the prevalence of a disease increases in a population, the positive predictive value increases and the negative predictive value decreases.
In summary, molecular analysis of FNA specimens is a useful adjunct in the evaluation of thyroid nodules for malignancy, which needs to be considered alongside the history, examination findings, thyroid function tests, ultrasound findings and FNA cytology. It is particularly useful when deciding the most appropriate management strategy for patients with indeterminate cytology (Bethesda III and IV) and may reduce the number of diagnostic hemithyroidectomies performed.
Special Populations
Pregnancy – thyroid nodules and goitres may grow significantly during pregnancy, and in some cases, cause airway obstruction by compressing the trachea. Thyroid nodules in pregnancy are evaluated the same way as in nonpregnant people – with thyroid function tests, an ultrasound and an ultrasound-guided FNA for selected nodules. Obviously, radionuclide thyroid scans are not performed during pregnancy.
Thyroid nodules in the setting of a multinodular goitre – an ultrasound scan in a patient with a goitre involving both thyroid lobes may be reported as showing multiple nodules. Some of these nodules may meet criteria for FNA due to their size and TI-RADS classification. If the patient is symptomatic, there is little to be gained by performing an ultrasound-guided FNA if they are to undergo surgery anyway (discussed below).
*This is a breakdown from a medical article published by Dr. Francis Hall. The third part of this article will be included in the next newsletter.